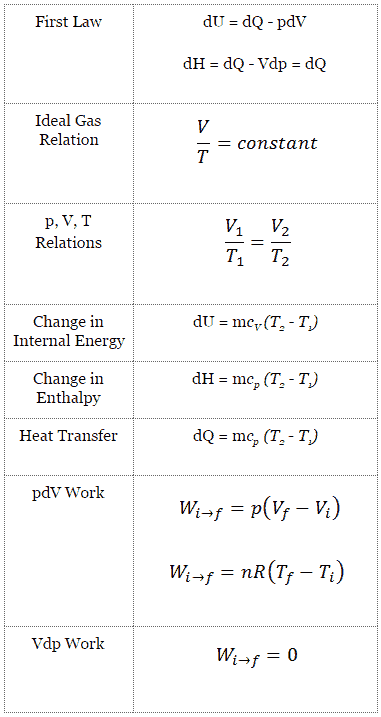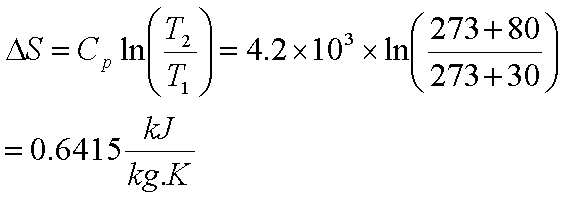When the volume of a system is constant changes in its internal energy can be calculated by substituting the ideal gas law into the equation for δu.
Change in specific internal energy formula.
To understand the relationship between work and heat we need to understand the factor of linking factors.
We cannot create nor destroy energy but we can convert or transfer it.
Internal energy refers to all the energy within a given system including the kinetic energy of molecules and the energy stored in all of the chemical bonds between molecules.
This is the change in internal energy.
Where and have been used to denote the specific heats for one kmol of gas and is the universal gas constant.
The specific heat ratio or is a function of only and is greater than unity.
Consider for example the following solved problem.
It keeps account of the gains and losses of energy of the system that are due to changes in its internal state.
In this manner doing some work externally or volume and temperature may both intensify but it will be made definite by the situations.
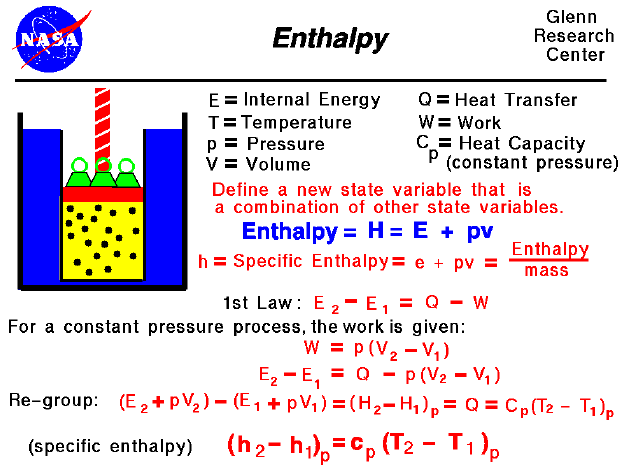
The heat flow is equal to the change in the internal energy of the system plus the pv work done.
The internal energy is measured as a difference from a.
Since specific internal energy is a property of the system it is usually presented in the property tables such as in the steam tables.
So that gives us that delta u or change in internal energy is negative 485 joules then if we plug this all into our calculator to calculate the work we get positive 25 25 joules.
An ideal gas with specific heats independent of temperature and is referred to as a perfect gas for example monatomic gases and diatomic gases at ordinary temperatures are considered perfect gases.
The change in the internal energy of a system is the sum of the heat transferred and the work done.
Internal energy formula is the heat energy stocked in gas.
The internal energy of a thermodynamic system is a measure of the energy within it excluding the kinetic energy of motion of the system as a whole and the potential energy of the system as a whole due to external force fields.
With the interactions of heat work and internal energy there are energy transfers and conversions every time a change is made upon a system.
For a temperature change at constant volume dv 0 and by definition of heat capacity d q v c v dt.
If a certain amount of heat is applied to gas the result is that the temperature of the gas may increase or else the volume of gas might increase.
So if we add our heat and our work here we get that the overall change in internal energy for this process is negative 460 joules.
Internal energy u the third component of our closed system energy equation is the change of internal energy resulting from the transfer of heat or work.
Specific energy or massic energy is energy per unit mass it is also sometimes called gravimetric energy density or just energy density though energy density more precisely means energy per unit volume it is used to quantify for example stored heat and other thermodynamic properties of substances such as specific.