2 form a group of 4 members each and choose among your group the person who will act as timer recorder leader and presenter.
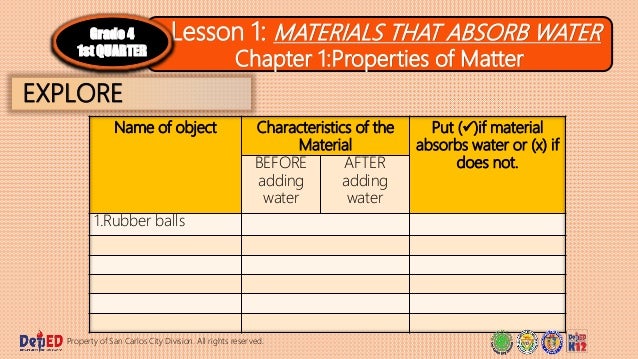
Characteristics of rubber ball after adding water.
Naphthalene is an organic compound with formula c 10 h 8 it is the simplest polycyclic aromatic hydrocarbon and is a white crystalline solid with a characteristic odor that is detectable at concentrations as low as 0 08 ppm by mass.
It is best known as the main ingredient of traditional mothballs.
Add1 2 teaspoon of rubber latex into the cup with vinegar.
1 the whole group will listen to the instructions given by the teacher.
4 stir with the spoon and try to form a glob of rubber into a ball.
Rubber ball cotton balls sponge face towel rug t shirt.
Each can from the same manufacturer can be different.
Each ball in a can is different in weight diameter and bounce.
Balls become softer with use.
While you wash the ball squeeze out any excess water from the latex ball and any bubbles of trapped vinegar inside the ball.
As an aromatic hydrocarbon naphthalene s structure consists of a fused pair of benzene rings.
Some balls are not very bouncy to begin with like squash balls.
Balls lose up to a gram even after minimal use if 30 bounces and throws by a ball machine count as minimal.
Make sure you are wearing the chemical splash goggles to avoid the possibility of vinegar coming into contact with the eye.
General purpose rubber qdrop comparison of rubber ball and hanenaito ball height rubber ball time hanenairo ball before impregnation after impregnation taber abrasion test results material test standard urethane super abrasion esistant vulkollan abrasion resistant urethane ceramic urethane abrasion test taber method 197 3 33 9 73 8 101.
Balls bounce differently on different axes.
In one cup add one tablespoon of vinegar and two spoons of water.
In one glass jar add three tablespoons of water and three tablespoons of elmer s glue.
It will be necessary to wash the ball in a sink with the water running or in a tub of water.
Once all the cotton balls are in the water refill the empty glass with more cotton balls.
If the ball is made of rubber then the stiffness argument above would incline me to predict that the ball will still be less bouncy when cold.
Some kinds of stiff materials do not dissipate energy very much like steel.
Ask your students if these will.
5 use the spoon and put the rubber ball in the cup from step 2 to wash it.
Keep count of the number of cotton balls as you add them to the water.
A steel ball on a steel floor is amazingly bouncy.